Abstract
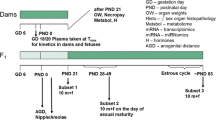
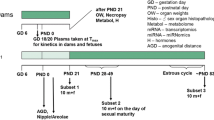
We performed an uterotrophic assay, the Hershberger assay, and a 28-day repeated-dose toxicity study (enhanced OECD test guideline No. 407) of 4,4´-[1-[4-[1-(4-hydroxyphenyl)-1-methylethyl]phenyl]ethylidene]bis[phenol] based on the OECD draft protocols. In the uterotrophic assay, female SD rats were subcutaneously injected with the chemical at doses of 0, 100, 300, and 1,000 mg/kg on each of 3 days from postnatal day 20 to day 22, and the uterine weight of rats given the 1,000 mg/kg dose of the test chemical plus ethinyl estradiol decreased. In the Hershberger assay, the test chemical was orally administered at doses of 0, 100, 300, and 1,000 mg/kg day to castrated male SD rats for ten consecutive days beginning on postnatal day 56, and no changes were observed. On the other hand, when the test chemical was orally administered at doses 0, 100, 300, and 1,000 mg/kg day for at least 28 days, a decrease in LH values in rats of both sexes and a decrease in FSH and estradiol values in female rats were detected in the 1,000 mg/kg group, and abnormal estrous cycles, uterine glandular atrophy, persistence of ovarian corpora lutea, vaginal epithelial mucification, and mammary glandular hyperplasia were also observed in one female rat in the 1,000 mg/kg group. Therefore, the uterotrophic assay used in this study showed that the chemical has the estrogen–antagonist properties, and some potentially endocrine-mediated effects were detected in growing rats based on the results of the enhanced OECD test guideline No. 407. However, the changes were observed in rats given a high dose of the chemical, 1,000 mg/kg day.
Similar content being viewed by others
References
Andrews P, Freyberger A, Hartmann E, Eiben R, Loof I, Schmidt U, Temerowski M, Becka M (2001) Feasibility and potential gains of enhancing the subacute rat study protocol (OECD test guideline no. 407) by additional parameters selected to determine endocrine modulation. A pre-validation study to determine endocrine-mediated effects of the antiandrogenic drug flutamide. Arch Toxicol 75:65–73
Cho SD, Kim JH, Kim DY, Lee YS, Kang KS (2003) Pre-validation study for OECD enhanced test guideline 407 protocol by gavage for 4 weeks using propylthiouracil and tamoxifen. Toxicol Lett 144:195–204
Kennel P, Pallen C, Barale-Thomas E, Espuna G, Bars R (2003) Tamoxifen: 28-day oral toxicity study in the rat based on the enhanced OECD test guideline 407 to detect endocrine effects. Arch Toxicol 77:487–499
Kunimatsu T, Yamada T, Miyata K, Yabushita S, Seki T, Okuno Y, Matsuo M (2004) Evaluation for reliability and feasibility of the draft protocol for the enhanced rat 28-day subacute study (OECD guideline 407) using androgen antagonist flutamide. Toxicology 2000:77–89
Mellert W, Deckardt K, Walter J, Gfatter S, van Ravenzwaay B (2003) Detection of endocrine-modulating effects of the antithyroid acting drug 6-propyl-2-thiouracil in rats, based on the “enhanced OECD test guideline 407”. Regul Toxicol Pharmacol 38:368–377
Miyata K, Shiraishi S, Houshuyama S, Imatanaka N, Umano T, Minobe Y, Yamasaki K (2006) Subacute oral toxicity study of di(2-ethylhexyl)adipate based on the draft protocol for the “enhanced OECD test guideline no. 407”. Arch Toxicol 80:181–186
OECD (1999) EMSG OECD draft proposal for testing of adequacy of an enhanced OECD protocol, repeated dose (28 days) toxicity (oral) study, based on OECD 407. OECD, Paris
OECD (2000) OECD protocol for investigating the efficacy of the enhanced TG407 test guideline (phase 2), rationale for the investigation, and description of the protocol. OECD, Paris
OECD (2001) Third meeting of the validation management group for the screening and testing of endocrine disrupters (mammalian effects). OECD, Paris
OECD (2006) OECD 5th meeting of the validation management group for mammalian effects testing (VMG-Mammalian) of the task force on endocrine disrupters testing AND assessment. OECD, Washington
Okazaki K, Imazawa T, Nakamura H, Furukawa F, Nishikawa A, Hirose M (2002a) A repeated 28-day oral dose toxicity study of 17α-methyltestosterone in rats, based on the ‘enhanced OECD test guideline 407’ for screening endocrine-disrupting chemicals. Arch Toxicol 75:635–642
Okazaki K, Okazaki S, Nakamura H, Kitamura Y, Hatayama K, Wakabayashi S, Tsuda T, Katsumata T, Nishikawa A, Hirose M (2002b) A repeated 28-day oral dose toxicity study of genisein in rats, based on the ‘enhanced OECD test guideline 407’ for screening endocrine-disrupting chemicals. Arch Toxicol 76:553–559
Okazaki K, Okazaki S, Nishimura S, Nakamura H, Kitamura Y, Hatayama K, Nakamura A, Tsuda T, Katsumata T, Nishikawa A, Hirose M (2001) A repeated 28-day oral dose toxicity study of methoxychlor in rats, based on the ‘enhanced OECD test guideline 407’ for screening endocrine-disrupting chemicals. Arch Toxicol 75:513–521
Shin JH, Moon HJ, Kim TS, Kang IH, Ki HY, Choi KS, Han SY (2006) Repeated 28-day oral toxicity study of vinclozolin in rats based on the draft protocol for the “enhanced OECD test guideline no. 407” to detect endocrine effects. Arch Toxicol 80:547–554
Shiraishi K, Miyata K, Houshuyama S, Imatanaka N, Umano T, Minobe Y, Yamasaki K (2006) Subacute oral toxicity study of diethylphthalate based on the draft protocol for “Enhanced OECD Test Guideline no. 407”. Arch Toxicol 80:10–16
Toyoda K, Shibuntani M, Tamaru T, Koujitani T, Uneyama C, Hirose M (2000) Repeated dose (28 days) oral toxicity study of flutamide in rats, based on the draft protocol for the ‘enhanced OECD test guideline 407’ for screening for endocrine-disrupting chemicals. Arch Toxicol 74:127–132
Wason S, Pohlmeyer-Esch G, Pallen C, Palazzi X, Espina G, Bars R (2003) 17α-methyltestosterone: 28-day oral toxicity study in the rat based on the “enhanced OECD test guideline 407” to detect endocrine effects. Toxicology 192:119–137
Yamasaki K, Noda N, Imatanaka N, Yakabe Y (2004) Comparative study of the uterotrophic potency of fourteen chemicals in a uterotrophic assay and their receptor-binding affinity. Toxicol Lett 146:111–120
Yamasaki K, Tago Y, Nagai K, Sawaki M, Noda S, Takatsuki M (2002a) Comparison of toxicity studies based on the draft protocol for the “enhanced OECD test guideline no. 407” and the research protocol of “pubertal development and thyroid function in immature male rats” with 6-n-propyl-2-thiouracil. Arch Toxicol 76:495–501
Yamasaki K, Sawaki M, Noda S, Imatanaka M, Takatsuki M (2002b) Subacute oral toxicity study of ethynyl estradiol and bisphenol A based on the draft protocol for the “enhanced OECD test guideline no. 407”. Arch Toxicol 76:65–74
Acknowledgments
This work was supported by a grant from Ministry of Economical Trade and Industry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamasaki, K., Miyata, K., Muroi, T. et al. Uterotrophic assay, Hershberger assay, and subacute oral toxicity study of 4,4´-[1-[4-[1-(4-hydroxyphenyl)-1-methylethyl]phenyl]ethylidene]bis[phenol] based on the OECD draft protocols. Arch Toxicol 81, 749–757 (2007). https://doi.org/10.1007/s00204-007-0211-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-007-0211-8