Abstract
The role of the genomic bipyrimidine nucleotide frequency in pyrimidine dimer formation caused by germicidal UV radiation was studied in three microbial reference organisms (Escherichia coli K12, Deinococcus radiodurans R1, spores and cells of Bacillus subtilis 168). The sensitive HPLC tandem mass spectrometry assay was used to identify and quantify the different bipyrimidine photoproducts induced in the DNA of microorganisms by germicidal UV radiation. The yields of photoproducts per applied fluence were very similar among vegetative cells but twofold reduced in spores. This similarity in DNA photoreactivity greatly contrasted with the 11-fold range determined in the fluence causing a decimal reduction of survival. It was also found that the spectrum of UV-induced bipyrimidine lesions was species-specific and the formation rates of bi-thymine and bi-cytosine photoproducts correlated with the genomic frequencies of thymine and cytosine dinucleotides in the bacterial model systems.
Similar content being viewed by others
Introduction
Ultraviolet (UV) disinfection is an environmentally safe method for inactivating microorganisms, especially as an alternative to chemical sterilization approaches where further reactions resulting in potentially noxious chemical complexes can occur (Chang et al. 1985; Blatchley et al. 2000; Eischeid and Linden 2007; Eischeid et al. 2009). Application of short wavelength UV radiation (UV-C) produced by low-pressure mercury lamps emitting mostly 254-nm (germicidal UV) is a standard practice for efficient and cost-effective disinfection of surfaces (Stannard et al. 1985), of air inside rooms (Nakamura 1987), and of water (Lazarova and Savoys 2004). UV sterilization is unmatched in its efficiency, simplicity, and dependability when applied as a microorganism disinfectant. The effectiveness of UV-C radiation as disinfectant arises primarily from the fact that DNA molecules absorb UV photons between 200 and 300 nm, with peak absorption around 260 nm, very close to the 254-nm radiation emitted by low-pressure mercury lamps (Douki and Cadet 2001; Sinha and Häder 2002; Douki et al. 2003; Cadet et al. 2005). Cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone photoproducts (6-4PPs) are the major photo-induced DNA lesions that are formed at DNA bipyrimidine sites in irradiated microorganisms, while strand breaks and DNA–protein-crosslinks are only produced in minor quantities (Harm 1980; Cadet et al. 2005 and references therein). In hydrated cells, both CPDs and 6-4PPs can be produced at any of the four bipyrimidine dinucleotides, although in different yields (Douki and Cadet 2001, 2003; Cadet et al. 2005). In dry DNA and DNA of bacterial endospores, UV-C induces predominantly 5,6-dihydro-5(α-thyminyl)thymine, the so-called spore photoproduct (SP) (Donnellan and Setlow 1965; Varghese 1970).
UV photoproducts within DNA are generally measured by two alternative approaches: (1) immunoassays using antibodies that specifically recognize CPDs and 6-4PPs as classes of bipyrimidine photoproducts (BPPs) and (2) chromatographic assays that simultaneously monitor a variety of photo-induced base lesions in DNA isolated from irradiated microorganisms (Cadet et al. 2005; Douki et al. 2005a, b; Pogoda de la Vega et al. 2005; Moeller et al. 2007a, b). The sensitive HPLC–MS/MS technique operating in the electrospray ionization (ESI) detection mode belongs to this last class. It has not only enabled the determination of the total amount of BPPs but also the identification and quantification of each individual type of DNA photoproduct formed in UV-irradiated microorganisms (Douki and Cadet 2001, 2003; Cadet et al. 2005; Matallana-Surget et al. 2008, 2009).
Recently, Matallana-Surget et al. (2008) made an interesting observation on the effect of the GC content of DNA on the distribution of UV-B induced BPPs. They reported that both the frequency and spectrum of BPPs was altered as a function of the genomic GC content. Broad spectrum UV-B radiation was applied to three different microorganisms, i.e. two marine bacteria, namely Sphingopyxis alaskensis RB2256 and Vibrio angustum S14, and the food-poisoning pathogen Salmonella typhimurium LT2. In addition to this set of microorganisms, they also irradiated commercially available DNA from Clostridium perfringens, calf thymus and Micrococcus luteus.
However, the overwhelming majority of published papers on the effects of UV on bacteria have been conducted (1) using germicidal monochromatic 254 nm UV radiation and (2) on genetically well-characterized microorganisms (Coohill and Sagripanti 2008, 2009 and references therein). Therefore, we followed up the interesting preliminary observation by Matallana-Surget et al. (2008) by using three of the well-characterized and most frequently tested microbial model systems in studying the microbial response to germicidal as well as environmentally relevant UV radiation, i.e. Escherichia coli K12, Deinococcus radiodurans R1, cells and endospores (spores) of Bacillus subtilis 168 (Nicholson and Galeano 2003; Pogoda de la Vega et al. 2005; Moeller et al. 2007a, c, 2009; Bauermeister et al. 2009). These bacteria have often been used as surrogates for pathogenic and food-poisoning bacteria such as B. anthracis and B. cereus (Nicholson and Galeano 2003; Weber et al. 2003) or Escherichia coli O157:H7 (Oguma et al. 2001), as well as UV biodosimetry surrogates for drinking water contaminants (Chang et al. 1985; Marshall et al. 2003; Deller et al. 2006).
In this short communication, we report on the impact of the genomic frequencies of bipyrimidine nucleotide combination on the formation of DNA photoproducts induced by germicidal 254-nm UV-C radiation. The UV-C induced BPPs were quantified by an HPLC–MS/MS approach. Emphasis was placed in this study on the quantitative characterization and discrimination of UV-C induced DNA base photolesions. Besides survival data, we present the spectrum of induced BPPs encountered within the DNA of the irradiated cells and discuss the role of genomic pyrimidine dinucleotide frequencies on the specificity of 254 nm UV-induced DNA photoproducts to strengthen the findings on the impact of GC content on the induction and distribution of BPP earlier reported by Matallana-Surget et al. (2008) after exposure to environmentally relevant polychromatic UV-B radiation.
Materials and methods
Bacterial strains, media and culturing conditions
B. subtilis 168 (DSM 402), D. radiodurans R1 (DSM 20539), E. coli K12 (DSM 498) were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ) GmbH, Braunschweig, Germany. Cells of B. subtilis and E. coli were cultivated under vigorous aeration at 37°C in nutrient broth (NB) medium (Difco, Detroit, MI) and D. radiodurans in tryptone-glucose-yeast (TGY) broth at 30°C as described previously (Oguma et al. 2001; Pogoda de la Vega et al. 2005; Moeller et al. 2007b, 2009). All vegetative cells were grown in liquid culture to mid-exponential growth phase, as monitored by OD measurement at 600 nm of 0.7 ± 0.1. For vegetative cells of B. subtilis, only suspensions with a spore fraction less than 0.01% were used, as determined by heat shock at 80°C for 15 min. Spores of B. subtilis were obtained by cultivation under vigorous aeration in double-strength liquid Schaeffer Sporulation Medium (Nicholson and Setlow 1990; Moeller et al. 2009), purified and stored as described previously (Moeller et al. 2007a, c, 2009).
UV-C irradiation
Cell suspensions in 25 ml of phosphate-buffered saline and spores in aqueous suspension at a concentration of 5 × 107/ml were exposed to 254 nm UV radiation emitted from a low-pressure mercury lamp (Model NN 8/15, Heraeus, Berlin, Germany). The spectral irradiance was determined spectrophotometrically (Bentham 150 double monochromator) and the fluence rate was 75.8 W/m2 as measured with a UV-X radiometer (UVP Ultra-Violet Products, Cambridge, UK) (Moeller et al. 2005; Pogoda de la Vega et al. 2005). Each irradiation was carried out at 4°C while the suspensions were continuously stirred to ensure homogeneous exposure (Pogoda de la Vega et al. 2005; Moeller et al. 2007a, c, 2009). To avoid photoreactivation by visible light, UV irradiation was performed in a black-painted photo-laboratory under yellow light at the German Aerospace Center, Institute for Aerospace Medicine, Cologne, Germany.
Quantification of BPPs
For DNA photoproduct analyses, chromosomal DNA was extracted from irradiated samples by using the Wizard Genomic DNA Purification Kit (Promega GmbH, Mannheim, Germany) procedure for genomic DNA isolation from bacteria (Pogoda de la Vega et al. 2005; Moeller et al. 2007a, c), whereas spores were chemically decoated prior DNA isolation as previously described by Sargent (1980). The extracted DNA was then enzymatically digested by nuclease P1, phosphodiesterases I and II and alkaline phosphatase, and dimeric photoproducts were released as modified dinucleoside monophosphates while normal bases were obtained as 2′-deoxyribonucleosides (Douki et al. 2005a, b; Pogoda de la Vega et al. 2005; Moeller et al. 2007a, c). BPPs that include CPDs [TT (thymine-thymine), TC (thymine-cytosine), CT (cytosine-thymine) and CC (cytosine-cytosine)] along with the corresponding 6-4PPs and related Dewar valence isomers (DEWs) as well as SP, all deriving from intrastrand photoreactions at bipyrimidine sites, were quantified by HPLC–MS/MS (Douki and Cadet 2001, 2003; Cadet et al. 2005).
Survival assay
Cell survival of the irradiated microorganisms was determined by assessing their ability to form macroscopic visible colonies (i.e. colony forming units, CFU) on nutrient agar plates after incubation under optimal growth conditions as described previously (Pogoda de la Vega et al. 2005; Moeller et al. 2007a, c).
Numerical and statistical analysis
Best-fit curves were used to calculate characteristic inactivation values for statistical comparison (Pogoda de la Vega et al. 2005; Moeller et al. 2007a, c). To determine the survival curve parameters, the following relationship was used: ln(N/N 0) = −k i × F + n, with N = CFU/ml after irradiation, N 0 = CFU/ml without irradiation, k i = inactivation constant (m2/J), F = UV fluence (J/m2) and n = extrapolation number, i.e. the intercept with the ordinate of the extrapolated semi-logarithmic straight line. The constants k i and n were determined by linear regression. Photoproduct induction curves were obtained by plotting the total number of BPPs per 104 bases versus fluence. The efficiency of photoproduct induction was determined from PP = kPP × F, with PP = BPPs/104 bases, kPP = photoproduct induction constant (BPPs/104 bases per J/m²). kPP was determined by linear regression of the induction curves (Pogoda de la Vega et al. 2005; Moeller et al. 2007a, c). The response of microorganisms to UV radiation was described by reporting the F10-value, which is defined as the UV fluence which reduces cell viability by a factor of 10 (Nicholson and Galeano 2003; Moeller et al. 2009). To measure intrinsic differences in the UV-C induced inactivation, all strains were treated in parallel under identical conditions of growth, irradiation, and survival testing. All experiments were repeated four times. The data shown are mean values ± standard deviation. The significance of the differences in the survival rates, BPPs induction and distribution were determined by analysis of variance (ANOVA), using Analyse-it software (Analyse-It Software, Ltd., Leeds, UK). Values were evaluated in multigroup pairwise combinations, and differences with P values of ≤0.05 were considered statistically significant (Pogoda de la Vega et al. 2005; Moeller et al. 2007a, b, 2009).
Results and discussion
Inactivation rates by UV-C radiation
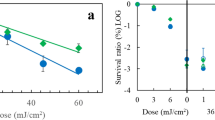
Figure 1 shows the characteristic survival curves of the different test systems as semi-logarithmic plots of the survival fraction versus UV fluence. Strictly exponential inactivation was obtained for E. coli and B. subtilis, whereas shoulder curves were obtained for D. radiodurans (Fig. 1a). F10-values were derived from these fluence-effect curves of inactivation (Table 1). Examination of the F10-values showed that E. coli was the most UV-sensitive microorganism (F10 = 23 ± 5 J/m2). The UV-resistance of the test systems increased in the following order: E. coli–B. subtilis cells–B. subtilis spores–D. radiodurans (Table 1). D. radiodurans (F10 = 603 ± 59 J/m2) was approximately 3 times more resistant to UV-C radiation than B. subtilis spores (F10 = 221 ± 38 J/m2), while vegetative cells of B. subtilis (F10 = 53 ± 8 J/m2) were fourfold more sensitive than their spore counterparts (Table 1). These F10 values of the UV-irradiated microorganisms were in good agreement with published data by various authors (Nicholson et al. 2000; Oguma et al. 2001; Nicholson and Galeano 2003; Pogoda de la Vega et al. 2005; Moeller et al. 2007a, c).
Fluence-survival (open symbols; a) and induction of BPPs (filled symbols; b) of D. radiodurans (circles), E. coli (squares), B. subtilis cells (triangles down) and spores (triangles up) after exposure to UV-C radiation. Data are averages ± standard deviation (n = 4). Otherwise error bars for survival data not shown were smaller than the symbol
Induction of BPPs
While survival determined from CFU is an indicator of the responses of whole cells to UV-C radiation, measurement of BPPs provides data on the molecular effects of UV-C radiation on DNA in the irradiated microorganisms. Since inactivation of microorganisms by UV-C radiation is effected through the formation of DNA lesions, a comparison of CFU and BPPs data may help to delineate UV biological consequences on the whole cell from its effects on the DNA (Setlow and Carrier 1966; Harm 1980; Sinha and Häder 2002; Cadet et al. 2005). For all three tested bacterial model systems, a linear increase in the total amount of BPPs per 104 bases with the applied fluence was found (Fig. 1b). The formation of BPPs, as determined from their PP induction constant (kPP), is ranged according to the microorganisms in the following increasing order: B. subtilis spores < D. radiodurans < B. subtilis cells < E. coli. However, the differences in kPP between vegetative cells of B. subtilis, D. radiodurans and E. coli were statistically not significant, whereas the kPP of the B. subtilis spores was twofold lower than those of the vegetative cells, as shown in the ratio of the total kPP values (Table 1). When comparing the different test systems with regard to their rates of UV-survival and BPPs formation, we found dramatic differences (up to 11.5 times) in the survival, but only weak differences in the induction of BPPs (Table 1).
Spectrum of the UV-C induced BPPs
CPDs were found to be the major lesions in all UV-C treated vegetative cells; however, significant differences in the fraction of the major and minor induced DNA photoproducts were detected between the different cellular systems (Table 2). In the DNA of UV-C irradiated D. radiodurans, the CPD TC was the major lesion and its fraction (47.2 ± 6.3%) amounted to almost half of all BPPs, while CPD TT (25.4 ± 5.1%), CPD CT (14.6 ± 3.4%), CPD CC (7.3 ± 1.7%) and 6-4 TC (6.2 ± 1.9%) were formed in moderate yields. The major UV-C induced BPPs in E. coli were CPD TC (38.3 ± 6.2%) and CPD TT (34.5 ± 3.1%) in almost equal quantities, while 6-4 TC (10.8 ± 3.8%) and CPD CT (10.2 ± 2.0%) were formed at a moderate rate. The major photolesions in vegetative B. subtilis cells were CPD TT (53.1 ± 3.2%) and CPD TC (21.1 ± 4.3%), while CPD CC (3.2 ± 0.7%) and 6-4 TC (15.7 ± 3.8%) were formed in moderate quantities. CPD CT and 6-4 TT add up to 2% of the total BPPs and slight traces of DEW TC (0.4 ± 0.1%), corresponding to approximately 10 DEW TC photolesion/109 bases per J/m2, were detected (Table 2). SP was only found in the UV-C treated B. subtilis spores, representing the major photoproduct (93.7 ± 5.6%) that is formed at the expense of minor CPD TC (2.7 ± 0.8%), CPD TT (2.4 ± 0.7%), 6-4 TC (3.8 ± 1.2%), and 6-4 TT (0.4 ± 0.1%). These results are in good agreement with previous observations (Douki et al. 2005a, b; Moeller et al. 2007a, b). It is clearly seen that the spectrum of BPPs formed in the DNA of UV-C irradiated dormant spores is significantly different from that of vegetative B. subtilis cells (Table 2). This unique photochemistry of bacterial endospores is mainly based on the A-DNA helix structure that is predominant during sporulation (Nicholson et al. 2000, 2005; Setlow 2006 and references therein). In all measured DNA BPPs, only minor levels of Dewar valence isomers (DEW TT and TC) were detected.
Relationship between decimal reduction of survival (F10-values) and induced BPPs
The data in Fig. 2 shows the number of UV-C induced BPPs that were required to reduce the survival of the different test systems by one order of magnitude, i.e. at the respective F10-value (Fig. 2). To obtain the same level of inactivation, different amounts of BPPs were required by the test systems. Approximately 53,720 ± 5,290 BPPs were necessary for the inactivation of 90% of the initial D. radiodurans cells, whereas spores of B. subtilis required about 5 times fewer BPPs (10,742 ± 1,852) for the same level of reduction, and cells of B. subtilis and E. coli up to 12 times fewer BPPs (7,155 ± 1,085 and 4,328 ± 974, respectively) for reaching their respective F10 values.
Relationship between the fluence causing decimal reduction of survival (F10 value) and the number of BPPs (in BPPs/104 bases per J/m2) at the individual F10 value in D. radiodurans (circles), E. coli (squares), B. subtilis cells (triangles down) and spores (triangles up). The regression on a straight line was determined by the least-squares method for four independent series of experiments. Coefficient of determination (r 2) was 0.942 for four data sets. Data are reported as averages and standard deviations (n = 4)
When assuming that UV radiation damages randomly the DNA in UV-exposed cell(s) and each microbial system, i.e. single cell (E. coli and B. subtilis) or spore (B. subtilis) (Nicholson et al. 2005), contains at least one genomic unit, one can calculate the mean frequency of UV-induced BPPs in the DNA for the F10-value of each microorganism. It follows that on the average one BPP was formed every 392 ± 67 bp in B. subtilis spores, every 589 ± 88 bp in B. subtilis cells and every 1,072 ± 219 bp in E. coli cells for UV-C fluences corresponding to 10% survival. Genomic information on the number of chromosomes and plasmids of the investigated microbial model systems was used according to the TIGR database (http://cmr.tigr.org (Peterson et al. 2001)). In short, B. subtilis 168 has a genome size of 4,214,814 bp, D. radiodurans R1 with two chromosomes (chromosome I: 2,648,638 bp and chromosome II: 412,348 bp), a megaplasmid (megaplasmid MP1: 177,466 bp), a small plasmid (plasmid CP1: 45,704 bp), creating a genome size of total 3,284,156 bp for D. radiodurans R1, and E. coli K12 has a single chromosome with 4,639,221 bp. However, the situation is somewhat different for UV-C irradiated D. radiodurans cells because >95% of cells were present as tetrads, and each cell of D. radiodurans is estimated to contain approximately four chromosomes (Hanson 1978; Tirgari and Moseley 1980). In that case, at the F10 value, each single coccus of a D. radiodurans tetrad showed on average one BPP every 245 ± 24 bp. Comparing these values with the individual genome size, at 90% inactivation, approximately 1.6 ± 0.2% of the total bp of D. radiodurans were damaged by irradiation, whereas an amount of 0.09 ± 0.02% base dimerization of the total bp of E. coli led to decimal reduction caused by UV exposure. When comparing cells and spores of B. subtilis at their F10 value, their genomes are 0.17 ± 0.03% and 0.26 ± 0.04%, respectively dimerized by UV-C when their survival is reduced by one order of magnitude. Clearly, these results we obtained in this study show a linear relationship between the fluence causing a decimal reduction of survival (F10 value) and the total number of BPPs induced in the DNA of the irradiated microorganisms (Fig. 2). Concluding, the formation and distribution of BPPs in microbial DNA is primarily responsible for UV-C inactivation.
Correlations between genomic GC, C and T content and the BPPs induction
In our study, we used the frequency of pyrimidine dinucleotides in the published genomic sequences of the three test microorganisms, available at the NCBI “Complete Microbial Genomes” database (http://www.ncbi.nih.gov/genomes/lproks.cgi), to determine to dependency of the individual genomic bipyrimidine frequencies on the yield of BPPs upon UV-C irradiation. In Table 3, the frequencies of bipyrimidine dinucleotides (TT, CC, TC, CT) of the three bacterial species to their corresponding GC are shown. When analyzing the genomic bipyrimidine frequencies of the three bacterial species, it was observed that D. radiodurans has a relatively high CC (8.92%) and a low TT (4.02%) dinucleotide frequency; in contrast, the genome of B. subtilis contains a relatively high TT (9.89%) and low CC (4.60%) frequency (Table 3). E. coli is situated between B. subtilis and D. radiodurans with a genomic CC and TT dinucleotide frequency of 5.86 and 7.32%, respectively (Table 3). Only minor differences were observed in the TC (5.76–6.58%) and CT (5.09–6.04%) dinucleotide frequency ranges between the three investigated species (Table 3). In Fig. 3, the level of induced BPPs as a function of the genomic bipyrimidine nucleotide frequency of the three bacterial species is depicted, for vegetative cells of all bacterial species and in brackets for B. subtilis spores, used here in this work as one of the most used biodosimetric system for measuring microbial response to UV radiation (Munakata 1981; Munakata et al. 2000; Marshall et al. 2003). It should be kept in mind that even when spores of B. subtilis were often used as surrogate for other spore-forming Bacillus species, e.g. B. cereus and B. anthracis, there are several differences in the strain-specific spore resistance (Sagripanti et al. 2007; Carrera et al. 2008). However, similar response to UV-C resistance of spores of various Bacillus species, among those B. subtilis and B. anthracis, were reported recently (Nicholson and Galeano 2003; Coohill and Sagripanti 2008 and references therein).
Level of induced BPPs (TT-photoproducts, a; CC-photoproducts, b; TC-photoproducts, c; CT-photoproducts, d) as a function of the genomic bipyrimidine nucleotide frequency of D. radiodurans R1 (filled circles), E. coli K12 (filled squares), B. subtilis 168 cells (filled triangles down) and spores (filled triangles up; in brackets). Coefficient of determination (r 2) was 0.953 (for TT-photoproducts), 0.991 (for CC-photoproducts), 0.949 (for TC-photoproducts), and 0.179 (for CT-photoproducts) for three data sets (obtained from D. radiodurans R1, E. coli K12 and B. subtilis 168 cells). Data are reported as averages and standard deviations (n = 4). Data are averages ± standard deviation (n = 4). Otherwise error bars for survival data not shown were smaller than the symbol
Furthermore, spores of B. subtilis were used as reference organisms and highly resistant, metabolically inactive cell state (reviewed in Nicholson et al. 2000, 2005; Setlow 2006) in this study to present a broader spectrum on the microbial response to UV-C radiation. The preponderance of SP in the DNA of UV-irradiated spores can be explained by different factors including the dehydrated state of the spore core, the presence of large amounts of dipicolinic acid, and the binding of small, acid-soluble spore proteins (SASP) of the α/β-type to DNA (Douki et al. 2005a, b; Moeller et al. 2007a; Setlow 2006). Binding of α/β-type SASP to the DNA of spores, together with spore core dehydration, induces a change in the helical conformation of spore DNA from the B-form to an A-like form, which in turn alters its UV-C photochemistry to favor production of SP (Nicholson et al. 2000, 2005; Setlow 2006). UV-C DNA damage induced in bacterial spores is repaired during germination, when spores reactivate and return to vegetative growth (reviewed in Setlow 2006). Studies with mutant spores have identified two DNA repair systems involved at this stage: an SP-specific repair enzyme called SP lyase that cleaves SP back to the two original thymine residues in a monomerization reaction and the nucleotide excision repair (NER) pathway that can excise SP and fill in the resulting single-strand gap (Nicholson et al. 2000, 2005). Three enzymes forming the UvrABC system initiate NER of a variety of structurally different DNA lesions, mainly BPPs caused by UV radiation exposure. UvrB (the subunit B of the excinuclease complex ABC), the central component of this system, is responsible for the ultimate DNA damage recognition and participates in the incision of the damaged DNA strand (Au et al. 2005; Goosen and Moolenaar 2008).
In bacteria, a large number of different repair mechanisms have evolved to repair UV-induced DNA lesions. These mechanisms include direct reversal of the damage by a photolyase (photoreactivation), removing of the damaged base by a DNA glycosylase (base excision repair, BER), incision of the DNA adjacent to the damage by an endonuclease (UV-damage endonuclease, UVDE) or removal of a complete oligonucleotide containing the damage by NER (reviewed in Nicholson et al. 2000; Sinha and Häder 2002; Cadet et al. 2005; Goosen and Moolenaar 2008).
The BPPs formation rates correlated highly with genomic pyrimidine dinucleotide frequencies taking into consideration the well-documented photoreactivity of each of the four primary bipyrimidine sequences (Douki and Cadet 2001, 2003; Douki et al. 2003; Cadet et al. 2005; Matallana-Surget et al. 2008; 2009). In the case of significant differences in the dinucleotide frequency, i.e. TT and CC, it can be clearly shown that an increased TT (B. subtilis) and CC (D. radiodurans) bipyrimidine nucleotide frequency led to an increased formation of TT- and CC-photoproducts, respectively, compared to the other two bacterial species with lower TT- and CC-bipyrimidine nucleotide frequency (Fig. 3a, b). However, whereas clear significant tendencies in TT- and CC-photoproduct induction were obtained, no or only very weak correlation between TC- and CT-containing photoproducts and the respective corresponding TC and CT bipyrimidine nucleotide frequency can be determined (Fig. 3c, d), which is mainly due to the fact that non-significantly different TC and CT nucleotide frequency-containing bacterial species were investigated. The results obtained in this study raise some novel questions on the relationship between genome composition and UV-mutagenesis. It would be interesting to determine how variation in the bipyrimidine nucleotide frequencies of a genome is translated into number of bipyrimidine dinucleotides within actual genes. These data could be correlated with the distribution of DNA photolesions at the sequence level in a large series of species exhibiting different bipyrimidine nucleotide frequencies. Such information could provide some essential insight into mutagenesis caused by germicidal UV-C radiation and its consequences for the evolution of bacteria (Franklin and Haseltine 1986; Galhardo et al. 2007; Palmeira et al. 2006). Recently, Hendel et al. (2008) showed that specific BPPs such as the TT CPDs have significantly higher mutagenic properties when repaired via translesion DNA synthesis than do other BPPs, in agreement with previous data reported by Gentil et al. (1996). In chromosomal DNA from UV-C irradiated E. coli, Franklin and Haseltine (1986) could show CPDs are the major premutagenic lesions responsible for point mutations at sites of adjacent pyrimidine residues and were also responsible for induction of the SOS response leading to the repair and removal of this type of photolesions. Ongoing investigations on the mutagenic specificity of UV radiation-induced DNA lesions of the three microbial model systems are continuing to elucidate detailed insights into the microbial response to mutagenic events on (error-prone and error-free) DNA repair mechanisms.
References
Au N, Kuester-Schoeck E, Mandava V, Bothwell LE, Canny SP, Chachu K, Colavito SA, Fuller SN, Groban ES, Hensley LA, O’Brien TC, Shah A, Tierney JT, Tomm LL, O’Gara TM, Goranov AI, Grossman AD, Lovett SM (2005) Genetic composition of the Bacillus subtilis SOS system. J Bacteriol 187:7655–7666
Bauermeister A, Bentchikou E, Moeller R, Rettberg P (2009) Roles of PprA, IrrE, and RecA in the resistance of Deinococcus radiodurans to germicidal and environmentally relevant UV radiation. Arch Microbiol 191:913–918
Blatchley ER, Emerick RW, Hargy T, Hoyer O, Hultquist RH, Sakaji RH, Schmelling DC, Soroushian F, Tchobanoglous G (2000) Chapter 3: protocols. In: Melin G (ed) Ultraviolet disinfection, guidelines for drinking water and water reuse. National Water Research Institute, Fountain Valley, pp 37–56
Cadet J, Sage E, Douki T (2005) Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res 571:3–17
Carrera M, Zandomeni RO, Sagripanti JL (2008) Wet and dry density of Bacillus anthracis and other Bacillus species. J Appl Microbiol 105:68–77
Chang JCH, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG, Johnson JD (1985) UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol 49:1361–1365
Coohill TP, Sagripanti JL (2008) Overview of the inactivation by 254 nm ultraviolet radiation of bacteria with particular relevance to biodefense. Photochem Photobiol 84:1084–1090
Coohill TP, Sagripanti JL (2009) Bacterial inactivation by solar ultraviolet radiation compared with sensitivity to 254 nm radiation. Photochem Photobiol 85:1043–1052
Deller S, Mascher F, Platzer S, Reinthaler FF, Marth E (2006) Effect of solar radiation on survival of indicator bacteria in bathing waters. Cent Eur J Public Health 14:133–137
Donnellan JE Jr, Setlow RB (1965) Thymine photoproducts but not thymine dimers are found in ultraviolet irradiated bacterial spores. Science 149:308–310
Douki T, Cadet J (2001) Individual determination of the yield of the main UV-induced dimeric pyrimidine photoproducts in DNA suggests a high mutagenicity of CC photolesions. Biochemistry 40:2495–2501
Douki T, Cadet J (2003) Formation of the spore photoproduct and other dimeric lesions between adjacent pyrimidines in UVC-irradiated dry DNA. Photochem Photobiol Sci 2:433–436
Douki T, Laporte G, Cadet J (2003) Inter-strand photoproducts are produced in high yield within A-DNA exposed to UVC radiation. Nucleic Acids Res 31:3134–3142
Douki T, Setlow B, Setlow P (2005a) Effects of the binding of alpha/beta-type small, acid-soluble spore proteins on the photochemistry of DNA in spores of Bacillus subtilis and in vitro. Photochem Photobiol 81:163–169
Douki T, Setlow B, Setlow P (2005b) Photosensitization of DNA by dipicolinic acid, a major component of spores of Bacillus species. Photochem Photobiol Sci 8:591–597
Eischeid AC, Linden KG (2007) Efficiency of pyrimidine dimer formation in Escherichia coli across UV wavelengths. J Appl Microbiol 103:1650–1656
Eischeid AC, Meyer JN, Linden KG (2009) UV disinfection of adenoviruses: molecular indications of DNA damage efficiency. Appl Environ Microbiol 75:23–28
Franklin WA, Haseltine WA (1986) The role of the (6–4) photoproduct in ultraviolet light-induced transition mutations in E. coli. Mutat Res 165:1–7
Galhardo RS, Hastings PJ, Rosenberg SM (2007) Mutation as a stress response and the regulation of evolvability. Crit Rev Biochem Mol Biol 42:399–435
Gentil A, Le Page F, Margot A, Lawrence CW, Borden A, Sarasin A (1996) Mutagenicity of a unique thymine-thymine dimer or thymine-thymine pyrimidine pyrimidone (6–4) photoproduct in mammalian cells. Nucleic Acids Res 24:1837–1840
Goosen N, Moolenaar GF (2008) Repair of UV damage in bacteria. DNA Repair 7:353–379
Hanson MT (1978) Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol 134:71–75
Harm W (1980) Biological effects of ultraviolet radiation. Cambridge University Press, New York
Hendel A, Ziv O, Gueranger Q, Geacintov N, Livneh Z (2008) Reduced efficiency and increased mutagenicity of translesion DNA synthesis across a TT cyclobutane pyrimidine dimer, but not a TT 6–4 photoproduct, in human cells lacking DNA polymerase eta. DNA Repair 7:1636–1646
Lazarova V, Savoys P (2004) Technical and sanitary aspects of wastewater disinfection by UV irradiation for landscape irrigation. Water Sci Technol 50:203–209
Marshall MM, Hayes S, Moffett J, Sterling CR, Nicholson WL (2003) Comparison of UV inactivation of spores of three Encephalitozoon species with that of spores of two DNA repair-deficient Bacillus subtilis biodosimetry strains. Appl Environ Microbiol 69:683–685
Matallana-Surget S, Meador JA, Joux F, Douki T (2008) Effect of the GC content of DNA on the distribution of UVB-induced bipyrimidine photoproducts. Photochem Photobiol Sci 7:794–801
Matallana-Surget S, Douki T, Cavicchioli R, Joux F (2009) Remarkable resistance to UVB of the marine bacterium Photobacterium angustum explained by an unexpected role of photolyase. Photochem Photobiol Sci 8:1313–1320
Moeller R, Horneck G, Facius R, Stackebrandt E (2005) Role of pigmentation in protecting Bacillus sp. endospores against environmental UV radiation. FEMS Microbiol Ecol 51:231–236
Moeller R, Douki T, Cadet J, Stackebrandt E, Nicholson WL, Rettberg P, Reitz G, Horneck G (2007a) UV radiation induced formation of DNA bipyrimidine photoproducts in Bacillus subtilis endospores and their repair during germination. Int Microbiol 10:39–46
Moeller R, Stackebrandt E, Douki T, Cadet J, Rettberg P, Mollenkopf HJ, Reitz G, Horneck G (2007b) DNA bipyrimidine photoproduct repair and transcriptional response of UV-C irradiated Bacillus subtilis. Arch Microbiol 188:421–431
Moeller R, Stackebrandt E, Reitz G, Berger T, Rettberg P, Doherty AJ, Horneck G, Nicholson WL (2007c) Role of DNA repair by non-homologous end joining (NHEJ) in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV and ionizing radiation. J Bacteriol 189:3306–3311
Moeller R, Setlow P, Reitz G, Nicholson WL (2009) Roles of small, acid-soluble spore proteins and core water content in survival of Bacillus subtilis spores exposed to environmental solar UV radiation. Appl Environ Microbiol 75:5202–5208
Munakata N (1981) Killing and mutagenic action of sunlight upon Bacillus subtilis spores: a dosimetric system. Mutat Res 82:263–268
Munakata N, Makita K, Bolsee D, Gillotay D, Horneck G (2000) Spore dosimetry of solar UV radiation: applications to monitoring of daily irradiance and personal exposure. Adv Space Res 26:1995–2003
Nakamura H (1987) Sterilization efficacy of UV irradiation on microbial aerosols under dynamic airflow by experimental air conditioning systems. Bull Tokyo Med Dent Univ 34:25–40
Nicholson WL, Galeano B (2003) UV resistance of Bacillus anthracis spores revisited: validation of Bacillus subtilis spores as UV surrogates for spores of B. anthracis Sterne. Appl Environ Microbiol 69:1327–1330
Nicholson WL, Setlow P (1990) Sporulation, germination, and outgrowth. In: Harwood CR, Cutting SM (eds) Molecular biological methods for Bacillus. Wiley, Sussex, pp 391–450
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000) Resistance of bacterial endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64:548–572
Nicholson WL, Schuerger AC, Setlow P (2005) The solar UV environment and bacterial spore UV resistance: considerations for Earth-to-Mars transport by natural processes and human spaceflight. Mutat Res 571:249–264
Oguma K, Katayama H, Mitani H, Morita S, Hirata T, Ohgaki S (2001) Determination of pyrimidine dimers in Escherichia coli and Cryptosporidium parvum during UV light inactivation, photoreactivation, and dark repair. Appl Environ Microbiol 67:4630–4637
Palmeira L, Guéguen L, Lobry JR (2006) UV-targeted dinucleotides are not depleted in light-exposed prokaryotic genomes. Mol Biol Evol 23:2214–2219
Peterson JD, Umayam LA, Dickinson T, Hickey EK, White O (2001) The comprehensive microbial resource. Nucleic Acids Res 29:123–125
Pogoda de la Vega U, Rettberg P, Douki T, Cadet J, Horneck G (2005) Sensitivity to polychromatic UV-radiation of strains of Deinococcus radiodurans differing in their DNA repair capacity. Int J Radiat Biol 81:601–611
Sagripanti JL, Carrera M, Insalaco J, Ziemski M, Rogers J, Zandomeni R (2007) Virulent spores of Bacillus anthracis and other Bacillus species deposited on solid surfaces have similar sensitivity to chemical decontaminants. J Appl Microbiol 102:11–21
Sargent MG (1980) A procedure for isolating high quality DNA from spores of Bacillus subtilis 168. J Gen Microbiol 116:511–514
Setlow P (2006) Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525
Setlow RB, Carrier WL (1966) Pyrimidine dimers in ultraviolet-irradiated DNA’s. J Mol Biol 17:237–254
Sinha RP, Häder DP (2002) UV-induced DNA damage and repair: a review. Photochem Photobiol Sci 1:225–236
Stannard CJ, Abbiss JS, Wood JM (1985) Efficiency of treatments involving UV irradiation for decontaminating packaging board of different surface compositions. J Food Prot 48:786–789
Tirgari S, Moseley BEB (1980) Transformation in Micrococcus radiodurans: measurement of various parameters and evidence for multiple independently segregating genomes per cell. J Gen Microbiol 119:287–297
Varghese AJ (1970) 5-Thyminyl-5, 6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem Biophys Res Commun 38:484–490
Weber DJ, Sickbert-Bennett E, Gergen MF, Rutala WA (2003) Efficacy of selected hand hygiene agents used to remove Bacillus atrophaeus (a surrogate of Bacillus anthracis) from contaminated hands. JAMA 289:1274–1277
Acknowledgments
The authors express their sincere gratitude to Peter Heeg for the fruitful discussion and critical reading of the manuscript, and Jörg Nellen and Ulrike Pogoda de la Vega for their help in the data interpretation. This work was supported in part by grants from NASA (NNA06CB58G and NNX08AO15G) to W.L.N.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Moeller, R., Douki, T., Rettberg, P. et al. Genomic bipyrimidine nucleotide frequency and microbial reactions to germicidal UV radiation. Arch Microbiol 192, 521–529 (2010). https://doi.org/10.1007/s00203-010-0579-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-010-0579-3