Abstract
Background and purpose
It is not clear if prolongation of definitive external radiation therapy for prostate cancer has an effect on biochemical failure. The aim of this work was to evaluate whether the biologically effective dose (BED), and in particular the duration of radiotherapy, intended as overall treatment time, has an effect on biochemical failure rates and to develop a nomogram useful to predict the 6-year probability of biochemical failure.
Patients and methods
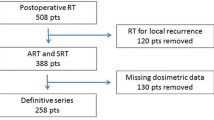
A total of 670 patients with T1–3 N0 prostate cancer were treated with external beam definitive radiotherapy, to a total dose of 72–79.2 Gy in 40–44 fractions. The computed BED values were treated with restricted cubic splines. Variables were checked for colinearity using Spearman’s test. The Kaplan–Meier method was used to calculate freedom from biochemical relapse (FFBR) rates. The Cox regression analysis was used to identify prognostic factors of biochemical relapse in the final most performing model and to create a nomogram. Concordance probability estimate and calibration methods were used to validate the nomogram.
Results
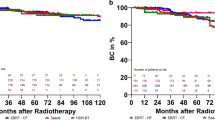
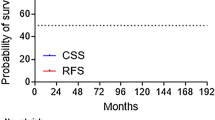
Neoadjuvant and concomitant androgen deprivation was administered to 475 patients (70 %). The median follow-up was 80 months (range 20–129 months). Overall, the 6-year FFBR rate was 88.3 %. BED values were associated with higher biochemical failure risk. Age, iPSA, risk category, and days of radiotherapy treatment were independent variables of biochemical failure.
Conclusion
A prolongation of RT (lower BED values) is associated with an increased risk of biochemical failure. The nomogram may be helpful in decision making for the individual patient.
Zusammenfassung
Hintergrund und Ziele
Es ist nicht geklärt, ob die Verlängerung einer definitiven Strahlentherapie bei der Behandlung von Prostatakarzinompatienten einen Effekt auf das biochemische Versagen hat. Die vorliegende Studie hat das Ziel zu evaluieren, ob biologisch die effektive Dosis und insbesondere die Gesamtdauer der Behandlung eine Wirkung auf das biochemisches Rezidiv haben könnte. Ferner wurde ein Nomogramm zur Vorhersage der 6-Jahres-Wahrscheinlichkeit von biochemischem Versagen entwickelt.
Patienten und Methoden
Insgesamt erhielten 670 Patienten im Tumorstadium T1–3, N0, eine Strahlentherapie mit einer Gesamtdosis von 72–79,2 Gy in 40–44 Fraktionen. Errechnete Werte der biologisch effektiven Dosis (BED) wurden mittels begrenzten kubischen Splines bearbeitet. Variablen wurden mit Spearman-Test hinsichtlich Kollinearität untersucht. Zur Abschätzung der Rate an „freedom from biochemical relapse” (Freiheit von biochemischen Versagen, FFBR) wurde die Kaplan-Meier-Methode angewendet. Mittels Cox-Regression wurden anhand des letzten am besten funktionierenden Models prognostische Faktoren für biochemisches Versagen identifiziert sowie ein Nomogramm etabliert. Durch Schätzung der Konkordanzwahrscheinlichkeit und mithilfe von Kalibrationsmethoden wurde das Nomogramm validiert.
Ergebnisse
Es erhielten 475 Patienten (70 %) neoadjuvant und begleitend eine Androgendeprivation. Der mediane Follow-up betrug 80 Monate (20–129). Insgesamt betrug die 6-Jahres-FFBR-Rate 88,3 %. BED-Werte waren mit höherem Risiko eines biochemischen Versagens assoziiert. Alter, initialer Wert des prostataspezifischen Antigens (PSA), Risikokategorie und Zeitraum der Strahlentherapie erwiesen sich als unabhängige Variable in Bezug auf biochemisches Versagen.
Schlussfolgerungen
Eine Verlängerung der Strahlentherapie (niedrige BED-Werte) ist mit einem steigenden Risiko eines biochemischen Versagen assoziiert. Das Nomogramm kann als Entscheidungshilfe in Bezug auf einzelne Patienten hilfreich sein.



Similar content being viewed by others
References
Duncan W, MacDougall RH, Kerr GR (1996) Adverse effect of treatment gaps in the outcome of radiotherapy for laryngeal cancer. Radiother Oncol 41:203–207
Chatani M, Matayoshi Y, Masaki N et al (1997) Radiation therapy for early glottic carcinoma (T1N0M0). Strahlenther Onkol 173:502–506
Manapov F, Klöcking S, Niyazi M et al (2012) Chemoradiotherapy duration correlates with overall survival in limited disease SCLC patients with poor initial performance status who successfully completed multimodality treatment. Strahlenther Onkol 188:29–34
D’Ambrosio DJ, Li T, Horwitz EM et al (2008) Does treatment duration affect outcome after radiotherapy for prostate cancer? Int J Radiat Oncol Biol Phys 72:1402–1407
Thames HD, Kuban D, Levy LB et al (2010) The role of overall treatment time in the outcome of radiotherapy of prostate cancer: an analysis of biochemical failure in 4839 men treated between 1987 and 1995. Radiother Oncol 96:6–12
Lai PP, Pilepich MV, Krall JM et al (1991) The effect of overall treatment time on the outcome of definitive radiotherapy for localized prostate carcinoma: the Radiation Therapy Oncology Group 75-06 experience. Int J Radiat Oncol Biol Phys 21:925–933
Zelefsky MJ, Leibel SA, Gaudin SB et al (1998) Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 41:491–500
Fowler JF (1989) The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62:679–694
Dale RG (1985) The application of the linear quadratic theory to fractionated and protracted radiotherapy. Br J Radiol 59:515–528
Williams SG, Taylor JMG, Liu N et al (2007) Use of individual fraction size data from 3756 patients to directly determine the ratio of prostate cancer. Int J Radiat Oncol Biol Phys 68:24–33
Miles EF, Lee WR (2008) Hypofractionation for prostate cancer: a critical review. Semin Radiat Oncol 18:41–47
Lukka H, Hayter C, Julian JA et al (2005) Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol 23:6132–6138
Yeoh EE, Fraser RJ, McGowan RE et al (2003) Evidence for efficacy without increased toxicity of hypofractionated radiotherapy for prostate carcinoma: early results of a phase III randomized trial. Int J Radiat Oncol Biol Phys 55:943–955
Barbieri V, Sanpaolo P, Genovesi D (2011) Interval between breast-conserving surgery and start of radiation therapy in early-stage breast cancer is not predictive of local recurrence: a single-institution experience. Clin Breast Cancer 11:114–120
Dale RG, Henfry JH, Jones B (2002) Practical methods for compensating for missed treatment days in radiotherapy, with particular reference to head and neck schedule. Clin Oncol 14:382–393
Roach M III, Hanks G, Thames H Jr et al (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65:965–974
Brenner DJ, Hall ET (1999) Fractionation and protraction of radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys 43:1095–1101
Hiratsuka J, Yoshimasa J, Yoshida K et al (2004) Clinical results of combined treatment conformal high dose rate iridium-192 brachytherapy and external beam radiotherapy using staging lymphadenectomy for localized prostate cancer. Int J Radiat Oncol Biol Phys 59:684–690
Harrell FE, Lee KL, Mark BD (1996) Multivariate prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Gonen M, Heller G (2005) Concordance probability and discriminatory power in proportional hazard regression. Biometrika 92:965–970
Horwitz EM, Vicini FA, Ziaja EL et al (1997) An analysis of clinical and treatment related prognostic factors on outcome using biochemical control as an end-point in patients with prostate cancer treated with external beam irradiaton. Radiother Oncol 44:223–228
Liauw SL, Liauw SH (2011) Prolongation of total treatment time because of infrequently missed days of treatment is not associated with inferior biochemical outcome after dose-escalated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 81:751–757
Miralbell R, Robert SA, Zubizarreta E et al (2012) Dose-fraction sensitivity of prostate cancer deduced from radiotherapy outcomes of 5969 patients in seven international institutional datasets: α/β = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 82:e17–e24
Sun L, Cabarcas SM, Farrar WL (2011) Radioresistance and cancer stem cells: survival of the fittest. J Carcinogene Mutagene S1:004. doi:10.4172/2157-2518.S1-004
Acknowledgments
The authors thank Dr. Loreta Sanpaolo for helping us with this manuscript and the editor and reviewers for their helpful comments.
Compliance with ethical guidelines
Conflict of interest
P. Sanpaolo, V. Barbieri, and D. Genovesi state that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Three possible examples of therapy interruption are described for a theoretical patient undergoing radiotherapy of 76 Gy in 38 fractions, 5 fractions per week, OTT of 52 days.
Example 1
His treatment starts on 18 November 2013 and should end on 8 January 2014, but because of interruptions on 25–26 December 2013, 1 and 6 January 2014 and included weekends, his treatment ends on 14 January, adding 6 days to his treatment (OTT 58 days).
On the basis of Eq. (2), BED = n•d•[1 + d/(α/β)]−[(0.693/α)•(T/Tpot)], the ideal BED is 153.5 Gy. The first break is after 27 fractions and 37 days (24 December 2013) with BED = 109 Gy (defined as BED pre-gap).
Eleven fractions in 21 days are still to be delivered with a BED of 41.7 Gy (defined as BED post-gap). The new BED value is therefore 109 + 41.7 = 150.7 Gy, with a difference of 2.8 Gy and 1.8 % of loss of dose. The BED value of 153.5 Gy has to be maintained so we introduce this equation:
BED pre-gap + BED post-gap = 153.5 Gy
BED = 27 • 2 • [1 + 2 / ( 1.5 ) ]− [(0.693/0.036)•(37/42)] + 11•d•[1 + d/(1.5)]−[(0.693/0.036)•(21/42)] = 153.5 Gy
where d is the new dose per fraction to be administered during the remaining 11 fractions in order to reach the ideal prescribed BED value.
The solution for d is d = 2.07 Gy. The new dose per fraction is not higher than the dose prescribed and the BED value could be easily compensated.
Example 2
The patient starts treatment on a Monday and breaks are required in weeks 5 and 6. The prescribed BED value is 153.5 Gy; OTT is 66 days.
The first break is after 20 fractions and 28 days with BED = 80.5 Gy (define as BED pre-gap). Thus, 18 fractions in 24 + 14 days are still to be delivered with a BED of 66.5 Gy (define as BED post-gap). The new BED value is therefore 80.5 + 66.5 = 147 Gy, with a difference of 6.5 Gy and 4.2 % of loss of dose.
Using the same equations of example 1, the new d value is d = 2.1 Gy. In this situation, the new dose per fraction is not higher than the dose prescribed and the BED value could be easily compensated.
Example 3
The patient starts on a Monday and is not able to complete the last eight fractions (1 week plus 3 days) that are administered after a 30-day interval. The prescribed BED value is 153.5 Gy; the OTT is 82 days.
The first break is after 30 fractions and 42 days with BED = 120.7 Gy (defined as BED pre-gap). Eight fractions in 10 + 30 days are still to be delivered with a BED of 19 Gy (defined as BED post-gap). The new BED value is therefore 120.7 + 19 = 139.7 Gy, with a difference of 13.8 Gy and 8.9 % of loss of dose.
Using the same equations of example 1, the new d value is d = 2.44 Gy. In this situation, the new dose per fraction becomes higher than the dose prescribed and the compensation of BED value should take into account surrounding normal tissue to PTV.
Rights and permissions
About this article
Cite this article
Sanpaolo, P., Barbieri, V. & Genovesi, D. Biologically effective dose and definitive radiation treatment for localized prostate cancer. Strahlenther Onkol 190, 732–738 (2014). https://doi.org/10.1007/s00066-014-0642-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0642-0