Abstract
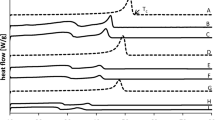
The fat obtained from nine commercial mar-garines purchased from Canada and the U.S.A. were crystallized from acetone at 15, 10, 5 and 0°C. The high melting triglyceride (HMG) fractions at 15°C contained high levels of palmitic and stearic acids. The 18:1 levels increased as fractionation temperature decreased. Triglyceride analysis re-vealed that the 11MG fractions contained high 1ev-els of carbon 54 and 52. The levels of trans iso-mers increased, whereas the trans levels in the 18:1 decreased with fractionation temperature. Mar-games made from canola oil exhibited β charac-teristics whereas canola-paim, soybean and corn margarines showed β1 crystals. The fractions as crystallized from acetone, showed numerous X-ray short spacings, characteristic of β1, β and in-termediate forms. Upon heating and cooling, the 15°C fraction showed β1 or a and β1 characteris-tics regardless of the polymorphic form present in the original margarines. The differential scan-ning calorimetry (DSC) melting points of these fractions varied from 53 to 50° C. The difference between the β and β1 margarines could be related to the 16:0 and carbon 54 content of the 15°C frac-tion. In the β tending margarines the 16:0 content was below 11%, in the β1 tending margarines above 17%. The carbon 54 content in the 15°C fraction of the β tending margarines was close to 70% and that of the β1 tending margarines around 50%. The triglyceride C54 in the 15°C fraction is β tending and therefore should be kept as low as possible. In canola margarines this can be achieved by in-corporation of palm oil, preferably in a slightly hydrogenated form.
Similar content being viewed by others
References
Sahasrabudhe, M.R., and C.J. Kurian,J. Inst. Can. Sci. Tech-nol. 12:140 (1979).
Carpenter, D.L., and H.T. Slover,J. Am. Oil Chem. Soc. 50:372 (1976).
Nazir, D.J., B.J. Moorecroft and M.A. Mishkel,Am. J. Clin. Nutr. 29:331 (1976).
Lo, L.C., and A.P. Handel,J. Am. Oil Chem. Soc. 60:815 (1983).
Parodi, P.W.,Aust. J. Dairy Sci. 3:20 (1974).
Sherbon, J.W., and R.M. Dolby,J. Dairy Sci. 56:52 (1973).
Jacobsberg, B.J., and O.C. Ho,J. Am. Oil Chem. Soc. 53:609 (1976).
Palmer, IS., and H.F. Wiese,J. Dairy Sci. 16:41 (1933).
Jenness, R., and L.S. Palmer,Ibid. 28:653 (1945).
Patton, S., and P.G. Kenney,Ibid. 41:1288 (1958).
Thompson, M.P., J.R. Brunner and CM. Stine,Ibid. 42:1651 (1959).
Wolf, D.P., and L.R. Dugan,J. Am. Oil Chem. Soc. 41:139 (1964).
Pi-Chen, C., and J.M. deMan,J. Dairy Sci. 49:617 (1966).
Persmark, I.L., K.A. Malkin and P.O. Stahl,Riv. Ital. Sost. Grasse. 53:301 (1976).
Yap, PH., M.Sc. thesis, University of Guelph.
Official and Tentative Methods of the American Oil Chemists’ Society, Vol. 1, Champaign, IL.
Shehata, A.A.Y., J.M. deMan and J.C. Alexander,Can. Inst. Food Sci. Technol. J. 3:85 (1970).
Shehata, A.A.Y., J.M. deMan and J.C. Alexander,Ibid. 4:61 (1971).
Mertens, W., and J.M. deMan,J. Am. Oil Chem. Soc. 49:366 (1972).
Postmus, E., L. deMan and J.M. deMan,Can. Inst. Food Sci. Technol. J. 22:481 (1989).
deMan, L., E. Postmus and J.M. deMan,J. Am. Oil Chem. Soc. 67:323 (1990).
Vaisey-Genser, M., and N.A.M. Eskin,Canola Oil: Properties and Performance, pp. 1–39, Canola Council of Canada, Winnipeg, Manitoba, 1982.
Weiss, T.J.,Food Oils and Their Uses, 2nd edn., AIV Westport, CT, 1983, p. 36.
Ward, J.,J. Am. Oil Chem. Soc. 65:1731 (1988).
Zalewski, S., and F.A. Kummerow,Ibid. 45:87 (1968).
Lutton, E.S., F.L. Jackson and Q.T. Quimby,J. Am. Chem. Soc. 70:2441 (1948).
Bailey, A.E., inMelting and Solidification of Fats, Interscience Publishers, Inc., New York, 1950, pp. 126–166.
Malkin, T., and B.R. Wilson,J. Chem. Soc. 369 (1949).
Timms, K.E.,Progress Lipid Res. 23:1 (1984).
Pryde, E.J., inHandbook of Soy Oil Processing and Utilization, edited by D.R. Erickson, E.H. Pryde, O.L. Brekke, T.L. Mounts and R.H. Falb, Publications American Soybean Association and American Oil Chemists’ Society 1980, pp. 13-30.
Moran, D.P.J.,J. Appl. Chem. 23:912 (1963).
Author information
Authors and Affiliations
Additional information
Presented at the Annual Meeting, Canadian Section of AOCS, October, 1989, Halifax, Canada.
About this article
Cite this article
D’Souza, V., deMan, L. & de Man, J.M. Chemical and physical properties of the high melting glyceride fractions of commercial margarines. J Am Oil Chem Soc 68, 153–162 (1991). https://doi.org/10.1007/BF02657760
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02657760