Abstract
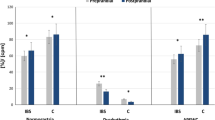
Regulatory neuropeptides are widely distributed in the gastrointestinal tract, where they play an important role in motility, secretion, and immune and inflammatory responses. In this study, the rectal mucosal content of somatostatin (SOM), substance P (SP), β-endorphin (BE), and thyrotropin-releasing hormone (TRH) was measured by radioimmunoassay in 56 patients with ulcerative colitis (UC), 15 patients with Crohn's disease (CD), 15 patients with acute infectious colitis (AIC), and 11 controls, who showed no inflammation of the rectal mucosa, nor abnormal bowel movements. The content of immunoreactive (ir)-SOM was decreased in UC patients, especially in those with persistent disease activity, while the levels of ir-SP, BE, and TRH were increased in such patients. Some changes of ir-peptide levels were also observed in CD and AIC patients. The changes in neuropeptide levels were analyzed in relation to histological grades of inflammation in UC patients, grades 4–5 showing the most significant changes. The levels of ir-SOM, SP, BE, and TRH showed no significant change in chronic persistent UC when measured 6–12 months after the initial examination. In contrast, in patients with remitting intermittent UC, the levels of SP and BE decreased during remission. Abnormal intestinal neuropeptide content may be implicated in the continued mucosal immune and inflammatory responses that are manifested in patients with inflammatory bowel disease.
Similar content being viewed by others
References
O'Dorisio MS. Neuropeptides and gastrointestinal immunity. Am J Med 1986;81(Suppl 6B):74–82.
Ottaway CA. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol Clin North Am 1991;20:511–529.
Mazumdar S, Das KM. Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am J Gastroenterol 1992;87:176–181.
Watanabe T, Kubota Y, Sawada T, et al. Distribution and quantification of somatostatin in inflammatory disease. Dis Colon Rectum 1992;35:488–494.
Kimura M, Masuda T, Hiwatashi N, et al. Changes in neuropeptide-containing nerves in human colonic mocosa with inflammatory bowel disease. Pathol International 1994;44:624–634.
Koch TR, Carney JA, Morris VA, et al. Somatostatin in the idiopathic inflammatory bowel diseases. Dis Colon Rectum 1988;31:198–203.
Calam J, Ghatei MA, Domin J, et al. Regional differences in concentrations of regulatory peptides in human colon mucosal biposy. Dig Dis Sci 1989;34:1193–1198.
Goldin E, Karmeli F, Selinger Z, et al. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig Dis Sci 1989;34:754–757.
Bernstein CN, Robert ME, Eysselein VE. Rectal substance P concentrations are increased in ulcerative colitis but not in Crohn's disease. Am J Gastroenterol 1993;88:908–913.
Mantyh PM, Catton M, Maggio JE et al. Alterations in receptors for sensory neuropeptides in human inflammatory bowel disease. Adv Exp Med Biol 1991;298:253–283.
Smith EM, Harbour-McMenamin D, Blalock JE. Lymphocyte production of endorphins and endorphin-mediated immunor-egulatory activity. J Immunol 1985;135:779S-782S.
Hart R, Wagner F, Steffens W, et al. Effect of thyrotropin-releasing hormone on immune functions of peripheral blood mononuclear cells. Reg Peptides 1991;27:335–342.
Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol Rev 1988; 40:121–162.
Taché Y, Garrick T, Raybould H. Central nervous system action of peptides to influence gastrointestinal motor function. Gastroenterology 1990;98:517–528.
Truelove SC, Witts LJ. Cortisone in ulcerative colitis. Final report on a therapeutic trial. BMJ 1955;2:1041–1048.
The Japanese Research Committee for Crohn's disease. Crohn's disease in Japan. Gastroenterol Jpn 1979;14:366–373.
Matts SGF. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med 1961;393–407.
Mitsuma T, Adachi K, Mukoyama M, at al. Concentrations of substance P-like immunoreactivity in the spinal cord of patients with amyotrophic lateral sclerosis. Med Sci Res 1987;15:1389–1390.
Kaneko H, Mitsuma T, Uchida K, et al. Immunoreactive somatostatin, substance P, and calcitonin gene-related peptide concentration of the human gastric mucosa in patients with nonulcer dyspepsia and peptic ulcer disease. Am J Gastroenterol 1993; 88:898–904.
Itoh M, Mitsuma T, Tomita A. Studies on the measurements of somatostatin by radioimmunoassay. Folia Endocrinol Jap 1977; 53:1106–1117.
Mitsuma T, Hirooka Y, Nihei N. Radioimmunoassay of thyrotropin-releasing hormone in human serum and its clinical application. Acta Endocrinol 1976;83:225–235.
Mitsuma T, Nogimori T Chaya M. Ethanol reduces plasma immunoreactive β-endorphin in rats. J Aichi Med Univ Assoc 1984;12:493–498.
Sundler F, Ekbald E, Hakanson R. Localization and colocalization of gastrointestinal peptides. In Brown DR (ed.) Handbook of experimental pharmacology. Vol. 106, gastrointestinal regulatory peptides. Berlin Heidelberg Tokyo New York: Springer, 1993;1–28.
Stanisz AM, Befus D, Bienenstock J. Differential effects of vasoactive intestinal polypeptide, substance P and somatostatin on immunoglobulin synthesis and proliferation by lymphocytes from Peyer's patches, mesenteric lymph nodes and spleen. J Immunol 1986;136:152–156.
Goetzl EJ, Payan DG. Inhibition by somatostatin of the release of mediators from human basophils and rat leukemic basophils. J Immunol 1984;133:3255–3259.
Adeyemi EO, Savage AP, Bloom SR, et al. Somatostatin inhibits neutrophil elastase release in vitro. Peptides 1990;11:869–871.
Koch TR, Carney JA, Go VLW Distribution and quantiation of gut neuropeptides in normal intestine and inflammatory bowel diseases Dig Dis Sci 1987;32:369–376.
Payan DC, Brewster DR, Goetzl EJ. Specific stimulation of human T lymphocytes by substance P. J Immunol 1983;131:1613–1615.
Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988;241:1218–1221.
Shanahan F, Demburg JA, Fox J, et al. Mast cell heterogeneity: Effect of neuroenteric peptides on histamine release. J Immunol 1985;135:1331–1337.
Ruff MR, Wahl SM, Pert CB. Substance P receptor-mediated chemotaxis of human monocytes. Peptides 1985;6[Suppl 2]:107–111.
Nakagawa N, Iwamoto I, Yoshida S. Effect of substance P on the expression of an adhesion molecule ICAM-1 in human vascular endothelial cells. Regul Peptides 1993;46:223–224.
Matis WL, Lavker RM, Murphy GF. Substance P induces the expression of an endothelial-leukocyte adhesion molecule by microvascular endothelium. J Invest Dermatol 1990;94:492–495.
Nakamura S, Ohtani H, Watanabe Y, et al. In situ expression of cell adhesion molecules in inflammatory bowel disease: Evidence of immunologic activation of vascular endothelial cells. Lab Invest 1993;69:77–85.
Malizia G, Calabrese A, Cottone M, et al. Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology 1991;100:150–159.
Gilman SC, Schwartz JM, Milner RJ, et al. β-endorphin enhances lymphocyte proliferative responses. Proc Natl Acad Sci USA 1982;79:4226–4230.
Bessler H, Sztein MB, Serrate SA. β-Endorphin modulation of IL-1-induced IL-2 production. Immunopharmacology 1990;19:5–14.
Hirooka Y, Ohtake K, Mitsuma T. Effects of glucocorticoid and β-endorphin on interleukin-2 production in human lymphocytes. J Aichi Med Univ Assoc 1988;16:547–555.
Puente J, Maturana P, Miranda D, et al. Enhancement of human natural killer cell activity by opioid peptides: Similar response to methionine, enkephalin and β-endorphin. Brain Behav Immun 1992;6:32–39.
Dennis E, Epps V, Saland L. β-Endorphin and met-enkephalin stimulate human peripheral blood mononuclear cell chemotaxis. J Immunol 1984;132:3046–3053.
Nikolopoulou V, Lafi T, Athanasiadou A, et al. Enhanced proliferative response to β-endorphin of phytohemagglutinin-activated lymphocytes from patients with ulcerative colitis. Z Gastroenterol 1991;29:283–285.
Leppäluoto J, Koivusalo F, Kraama R. Thyrotropin-releasing factor: Distribution in neural and gastrointestinal tissues. Acta Physiol Scand 1978;104:175–179.
Lersch C, Hammer C, Krombach F, et al. Effect of thyrotropin-releasing hormone (TRH) on the chemiluminescence (CL) activity of human mononuclear cells. J Clin Lab Immunol 1989; 28:69–71.
Pawlikowski M, Zerek-Melen G, Winczyk K. Thyroliberin (TRH) increases thymus cell proliferation in rats. Neuropeptides 1992;23:199–202.
Kunert-Radek J, Pawlikowski M, Stepien H, et al. Inhibitory effect of thyrotropin-releasing hormone on spontaneous proliferation of mouse spleen lymphocytes in vitro. Biochem Biophys Res Commun 1991;181:562–565.
Kruger TE, Smith EM, Blalock JE. Thyrotropin-releasing hormone (TRH) enhancement of in vitro antibody production. Fed Proc 1987;46:926.
Pawlikowski M, Zerek-Melen G, Winczyk K, et al. Comparison of the antiproliferative effects of colon mitosis inhibitor, thyroliberin and somatostatin analog octreotide on rat colonic mucosal epithelial cells. Cytobios 1993;73:25–30.
Skraastad Ø. Thyrotropin-releasing hormone (TRH) inhibits cell proliferation in large bowel epithelium and epidermis. Epithelia 1987;1:121–128.
Mitsuma T, Hirooka Y, Rhu R, et al. Somatostatin reduces plasma β-endorphin levels in rats. J Aichi Med Univ Assoc 1990;18:307–311.
Mitsuma T, Hirroka Y, Nakada K, et al. Effects of somatostatin on the release of immunoreactive β-endorphin from the rat stomach in vitro. J Aichi Med Univ Assoc 1988;16:29–34.
Mitsuma T, Hirooka Y, Nakada K. Somatostatin and cholecystokinin-8 inhibit thyrotropin-releasing hormone release from rat stomach in vitro. J Aichi Med Univ Assoc 1988;16:313–317.
Furusawa A, Morise K, Maeda Y, et al. Effect of luminal administration of thyrotropin-releasing hormone or somatostatin on gastric pH and interaction of these peptides in rat. Gastroenterol Jpn 1992;27:165–171.
Mitsuma T, Hirooka Y, Nakada K, et al. Effect of cholecystokinin-8 and somatostatin on thyrotropin-releasing hormone release from the rat cecum in vitro. J Aichi Med Univ Assoc 1989;17:769–774.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamamoto, H., Morise, K., Kusugami, K. et al. Abnormal neuropeptide concentration in rectal mucosa of patients with inflammatory bowel disease. J Gastroenterol 31, 525–532 (1996). https://doi.org/10.1007/BF02355052
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02355052