Conclusions
-
1.
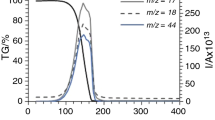
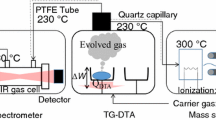
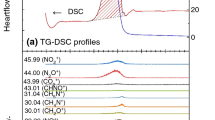
The composition of the gas phase during the thermal decomposition of pure ammonium perchlorate and ammonium perchlorate with an addition of 5% Fe2O3 at 400° and a pressure of 10–200 mm was investigated with a transit time mass spectrometer and by a procedure permitting recording of active particles and aggressive gases.
-
2.
A change in the composition of the gas phase during the reaction and an influence of the additive on the composition of the decomposition products were detected, which is explained by the role of the additive as a catalyst of reactions in the gas phase.
Similar content being viewed by others
Literature cited
O. P. Korobeinichev, V. V. Boldyrev, and Yu. Ya. Karpenko, Fizika Goreniya i Varyva,1, 33 (1968).
L. Dauerman, G. E. Salser, and Y. A. Tajima, A. I. A. A. Journal,11, 1501 (1967).
V. P. Strunin and E. L. Frankevich, Pribory i Tekhnika Eksperim.,2, 175 (1964).
J. B. Levy, J. Phys. Chem.,66, 1092 (1962).
S. H. Inami, W. A. Rosser, and H. Wise, J. Phys. Chem.,67, 1077 (1963).
O. P. Korobeinichev, Kinetika i Kataliz,5, 1169 (1968); O. P. Korobeinichev, V. V. Boldyrev, V. N. Pan'kov, and Yu. Ya. Karpenko, First All-Union Symposium on Combustion and Explosion, February 19–24, 1968, Summaries of Reports [in Russian], Nauka (1968), p. 191.
I. V. Davies, P. W. M. Jacobs, and A. Russel-Jones, Trans. Faraday Soc.,63, 1737 (1967).
J. N. Maycock, V. R. P. Verneker, and P. W. M. Jacobs, J. Chem. Phys.,46, 2857 (1967).
L. L. Bircumshaw and B. H. Newman, Proc. Roy. Soc.,A227, 228 (1955);A227, 115 (1954).
K. Kuratani, Aeronautical Res. Inst. Univ. Tokio, Report No.372, Tokio (1962), Part III.
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 4, pp. 778–781, April, 1969.
Rights and permissions
About this article
Cite this article
Korobeinichev, O.P., Boldyrev, V.V., Karpenko, Y.Y. et al. Investigation of rapid processes in the thermal decomposition of ammonium perchlorate with a transit time mass spectrometer. Russ Chem Bull 18, 706–708 (1969). https://doi.org/10.1007/BF00907026
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00907026